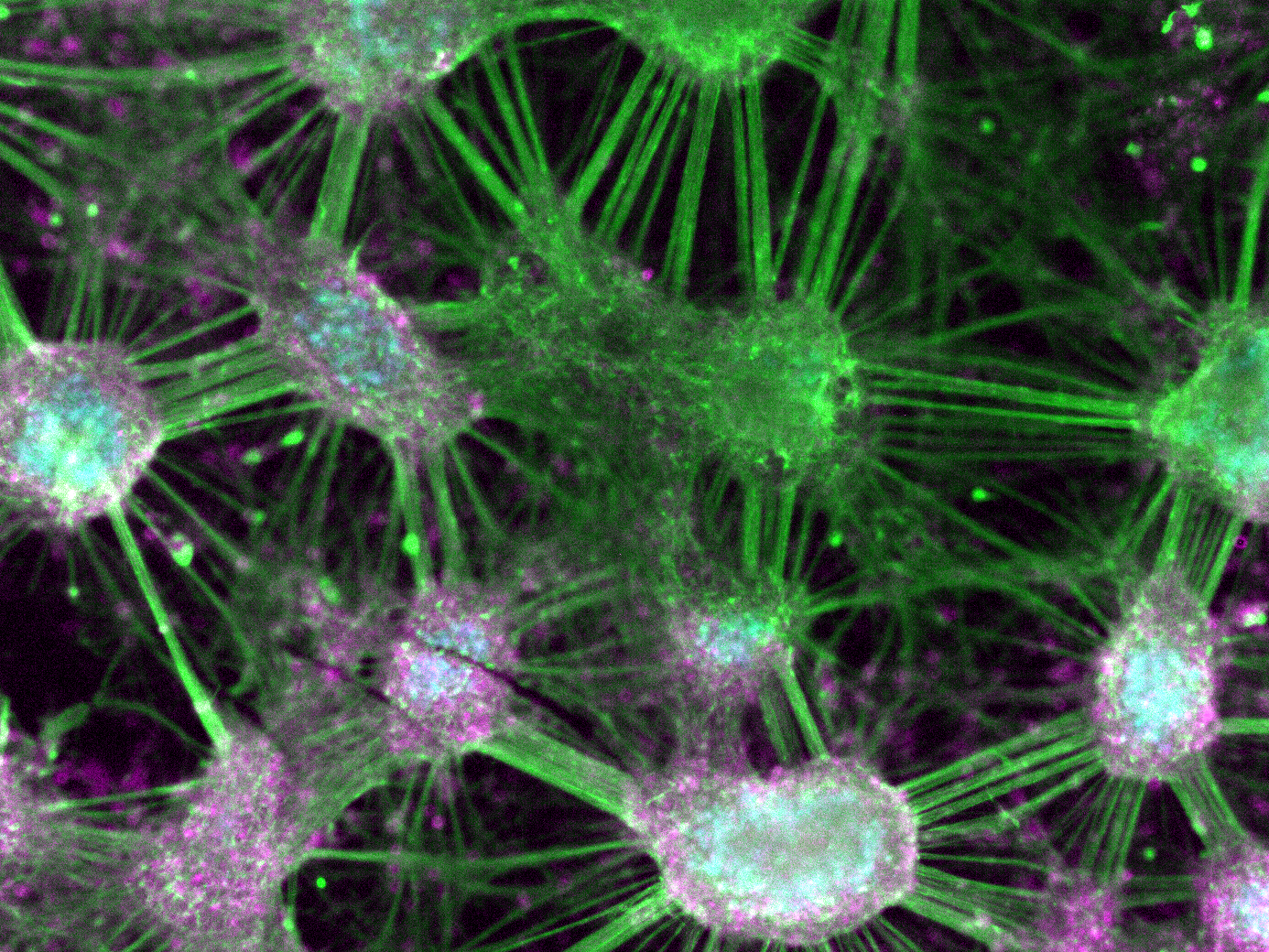
A glimpse into our research
Our general approach is to combine state-of-the-art imaging and single cell techniques with mechanistic modelling and bioinformatics analyses to investigate how the emergent behaviour of cells, organs and organisms originates from molecular entities.